The second law was formulated in the middle
of the last century by Clausius and Thomson following Carnot's
earlier observation that, like the fall or flow of a stream that
turns a mill wheel, it is the "fall" or flow of heat
from higher to lower temperatures that motivates a steam engine.
The key insight was that the world is inherently active, and that
whenever an energy distribution is out of equilibrium a potential
or thermodynamic "force" (the gradient of a potential)
exists that the world acts spontaneously to dissipate or minimize.
All real-world change or dynamics is seen to follow, or be motivated,
by this law. So whereas the first law expresses that which remains
the same, or is time-symmetric, in all real-world processes the
second law expresses that which changes and motivates the change,
the fundamental time-asymmetry, in all real-world process. Clausius
coined the term "entropy" to refer to the dissipated
potential and the second law, in its most general form, states
that the world acts spontaneously to minimize potentials (or equivalently
maximize entropy), and with this, active end-directedness or time-asymmetry
was, for the first time, given a universal physical basis. The
balance equation of the second law, expressed as S > 0, says
that in all natural processes the entropy of the world always
increases, and thus whereas with the first law there is no time,
and the past, present, and future are indistinguishable, the second
law, with its one-way flow, introduces the basis for telling the
difference.
The active nature of the second law is intuitively easy to grasp
and empirically demonstrate. If a glass of hot liquid, for example,
as shown in Figure 3, is placed in a colder room a potential exists
and a flow of heat is spontaneously produced from the cup to the
room until it is minimized (or the entropy is maximized) at which
point the temperatures are the same and all flows stop.
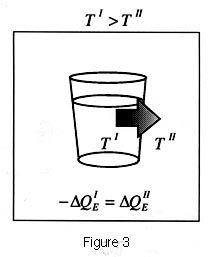
| A glass of liquid
at temperature TI is placed in a room at temperature
TII such that . The disequilibrium produces a field potential
that results in a flow of energy in the form of heat from the
glass to the room so as to drain the potential until it is minimized
(the entropy is maximized) at which time thermodynamic equilibrium
is reached and all flows stop. refers to the conservation of
energy in that the flow from the glass equals the flow of heat
into the room. (From Swenson, 1991a. Copyright 1991 Intersystems
Publications. Adapted by permission). |
Figure 4 shows various other potentials and
the flows they would produce. Of important theoretical interest
for this paper is the fact that Joule's experiment (Figure 2)
while designed to
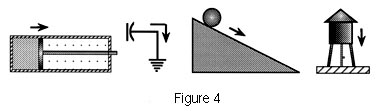
| Further examples
of potentials that follow from nonequilibrium distributions of
energy. Whenever energy (in whatever form) is out of equilibrium
with its surroundings, a potential exists for producing change
that, following the second law, is spontaneously minimized. |
show the first law unintentionally demonstrates
the second too. As soon as the constraint is removed the potential
produces a flow from the falling weight through the moving paddle
through the thermometer. This is precisely the one-way action
of the second law and the experiment depends upon it entirely.
The measurement of energy only takes place through the lawful
flow or time-asymmetry of the second law, and the point to underscore
is that the same is true of every measurement process. In addition,
every measurement process also a demonstrates the first law as
well since the nomological relations that hold require something
that remains invariant over those relations (or else one could
not get invariant or nomological results). The first and second
laws are thus automatically given in every measurement process
for the simple fact, in accordance with the discussion above,
that they are entailed in every epistemic act (Swenson, in press
a, b; see also Matsuno, 1989, in press on generalized measurement).
Here we begin to make the appropriate steps towards the a posteriori
recovery of the a priori given. It is just this reconciliation
that sets the tolerance space with respect to uniting the otherwise
incommensurable rivers and providing a principled account of the
epistemic dimension and the constitutive ecological relations
that instantiate it.
Boltzmann's View of the Second Law as a
Law of Disorder: A Fatal Problem for Ecological Relations
The active macroscopic nature of the second
law posed a direct challenge to the "dead" mechanical
world view which Boltzmann tried to meet in the latter part of
the last century by reducing the second law to a law of probability
following from the random collisions of mechanical particles (efficient
cause (see Swenson (1990)). Following the lead of Maxwell who
had modeled gas molecules as colliding billiard balls, Boltzmann
argued that the second law was simply a consequence of the fact
that since with each collision nonequilibrium distributions would
become increasingly disordered leading to a final state of macroscopic
uniformity and microscopic disorder. Because there are so many
more possible disordered states than ordered ones, he concluded,
a system will almost always be found either in the state of maximum
disorder or moving towards it.
As a consequence, a dynamically ordered state, one with molecules
moving "at the same speed and in the same direction,"
Boltzmann (1974/1886, p. 20) asserted, is thus "the most
improbable case conceivable...an infinitely improbable configuration
of energy." Because this idea works for certain near equilibrium
systems such as gases in boxes, and because science until recently
was dominated by near equilibrium thinking, Boltzmann's attempted
reduction of the second law to a law of disorder became widely
accepted as the second law rather than simply an hypothesis about
the second law, and one that we now know fails. It became the
apparent justification from physics for solidifying Cartesian
incommensurability and establishing the view of the two incommensurable
rivers-the "river" of biology, psychology, and culture,
or the epistemic dimension of the world characterized by intentional
dynamics and flowing up to increasingly higher states of order,
versus the "river" of physics flowing down to disorder.
Such a view is entirely inimical to a science of ecological relations,
since, as noted above, it is precisely through the interface of
these two rivers that these relations occur, and if the interface
is incommensurable then the relations are effectively prohibited,
or at best, incomprehensible.
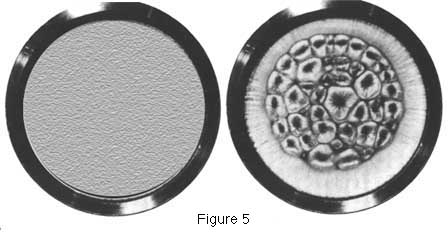
| Two time slices
from the Bénard experiment. When the gradient of the potential
(the "force") between source (the heated surface below)
and the sink (the cooler air at the top) is below a critical
threshold (left) the flow of heat is produced by the random collision
of the molecules (conduction), and the system is in the disordered
or "Boltzmann regime", and the surface of the system
is smooth, homogeneous, and symmetrical. When the force is above
the critical threshold (right), however, the symmetry of the
system is broken and autocatakinetic order spontaneously arises
as random microscopic fluctuations are amplified to macroscopic
levels and "Benard cells" fill the container as hundreds
of millions of molecules begin moving together (for more detailed
discussion see e.g., Swenson, 1989a,b, c, 1992, 1997a). (From
Swenson, 1989c. Copyright 1989 Pergamon Press. Used by permission). |


